Introduction: Ozurdex® (Allergan Pharmaceuticals, Castlebar Road, Westport, Ireland) is an intravitreal implant containing 0.7 mg dexamethasone. It is indicated in adult patients for the treatment of diabetic macular edema, cystoid macular edema due to central retinal vein occlusion, and in patients with non-infectious uveitis. Common complications after Ozurdex® administration include an increase in intraocular pressure, cataract progression or conjunctival suffusion. Acute retinal necrosis after Ozurdex® administration is a very rare and serious complication. According to our current research, this is the fourth published case. Extreme caution must be exercised when treating immunosuppressed patients with Ozurdex®.
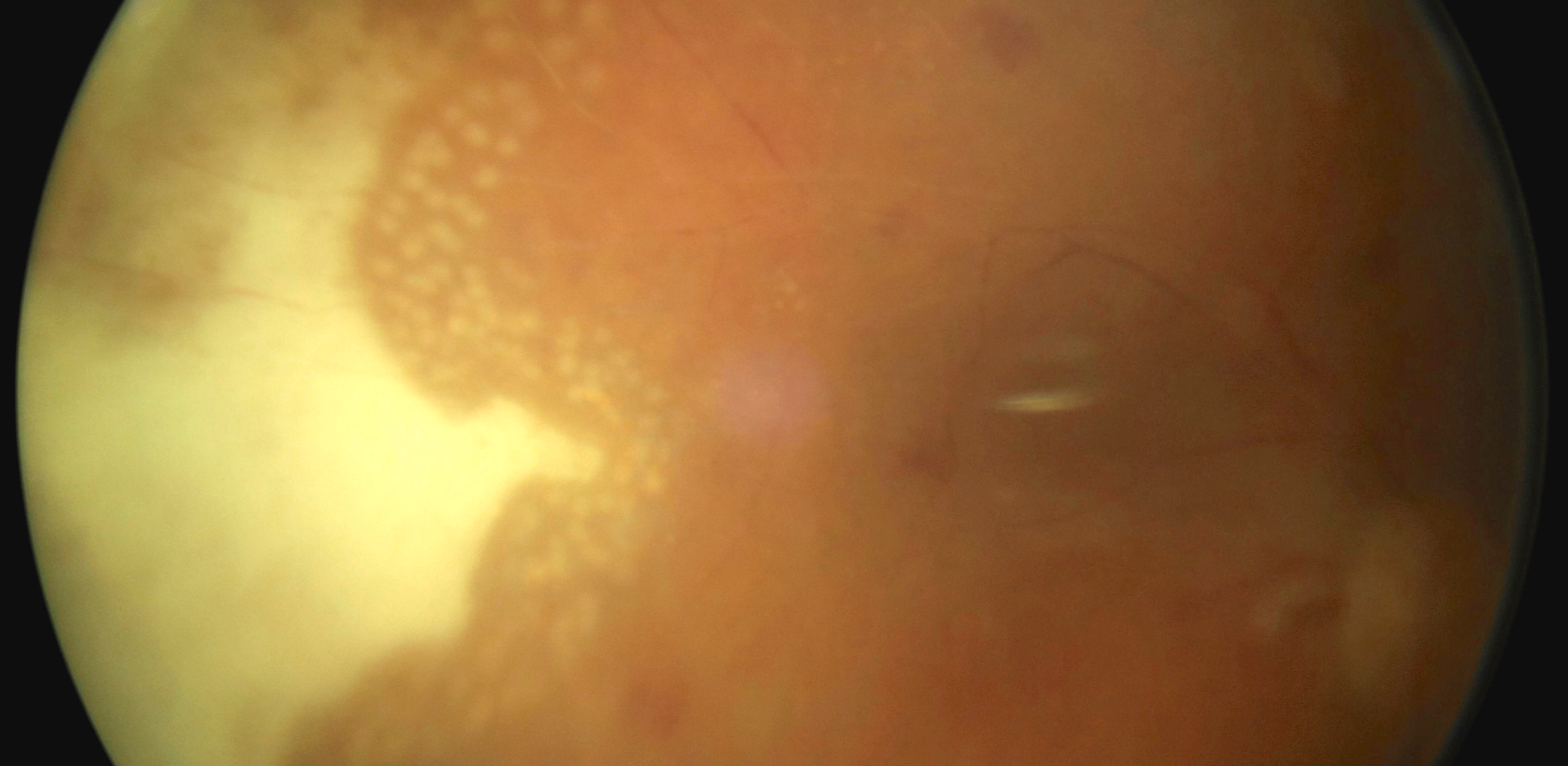
Case Report: This is a case report on an immunosuppressed 68-year-old patient with diabetic macular edema, who developed acute retinal necrosis 74 days after Ozurdex® implantation. He suffers from chronic myeloid leukemia and takes 400 mg cytostatic imatinib once per day. Urgent pars plana vitrectomy (PPV) with silicone oil instillation was performed and antiherpetic drugs were initiated intravenously. Serological examination confirmed an active infection of cytomegalovirus etiology (CMV).
Conclusion: Acute retinal necrosis is a rare necrotizing retinitis. Corticosteroids administered intravitreally reduce the local immune response, which may cause a primary infection or reactivation of a latent viral infection.